Biontex's Tips and Tricks for Successful Transfection Using Non-viral Chemical Transfection Methods
Non-viral transfection methods can be grouped into physical and chemical methods. Chemical transfection methods are based upon electrostatic bonding of nucleic acids with carrier constructs to form lipoplexes or polyplexes, depending on whether they derive from positively charged liposomes/lipids or polymers.
All chemical transfection methods show similar strengths and weaknesses as they follow a similar mechanism. Knowing this might help to avoid mistakes and improve results.
The following points are important for successful transfection with lipoplexes or polyplexes (and are discussed in more detail below):
Purity of nucleic acidsCell health
Proliferation of cells in plasmid transfection
Optimization parameter: Nucleic acid/reagent ratio and lipoplex/polyplex amount
Optimal measurement time
Construction of a successful transfection experiment
Adhesion to plastic surfaces and aging of lipoplexes
Up & downscaling
Stable transfection
The innate immune system
Freeze/thaw procedure
Edge effects
Microscope-FACS-GFP
Special case: Suspension cells
Comparison of experiments
Purity of nucleic acids
In principle, a high quality of the nucleic acids used should be taken into account. If synthetic constructs are used in transfection - as is often the case with siRNA, for example – the quality offered by most manufacturers is generally sufficient.
However, if cell-free biological systems, cell cultures or bacteria are used to synthesize the constructs, more attention must be paid to this topic.
On the one hand, care should be taken to avoid impurities with non-targeting nucleic acids, which can reduce the desired result or interfere with "off-target" effects.
In particular, however, contamination should be the focus of attention. This can be detected by the innate immune system of the cells - as is the case in plasmids produced in E.coli strains, for example.
If such plasmids are not free from lipopolysaccharides or endotoxins, transfection efficiency can be greatly reduced.
The innate immune system of many cell types is able to detect such endotoxins via toll-like receptor 4. A defense state occurs as a reaction, which makes transfection more difficult.
Purification of plasmids using the "miniprep" method does not sufficiently remove endotoxins. When cleaning kits are used, care must be taken that they meet the quality requirement of "endotoxin-free."

Cell health
For transfection to be successful, the cells must be healthy. While bacterial contamination can quickly lead to loss of cultures, fungi-based impurities, and mycoplasmas in particular, are often not recognized and remain latently present in the living cell culture.
However, the use of antibiotic agents such as penicillin / streptomycin to prevent bacterial contamination, promotes the possibility of contamination by microorganisms that are not killed by these antibiotics, such as fungi and mycoplasmas.
This reason for this is as follows. In our experience, cell culture products, that were formerly suspected of being an incidence gateway for mycoplasma or other contaminants - namely, serum and trypsin - are now safe when sourced from quality manufacturers.
Human sources and cross-contamination remain the main sources of contamination. Since selective contamination with mycoplasma or fungi is excluded, the best protection is to run cell culture without antibiotics.
For experienced staff, this is not a problem. However, if contamination occurs, this will immediately lead to visible bacterial growth and thus also to loss of culture.
Since most cell cultures are easily replaceable, this is not a big problem. Using antibiotics also reduces the risk of cross-contamination.
The risk of cross-contamination can be further reduced by taking various further actions. For example, the frozen cells can be stored in the gas phase over the liquid nitrogen in the cryogenic vessels instead of in the liquid nitrogen itself. Spatial and temporal separation of cell culture work from different cultures reduces the risk further. Nevertheless, monitoring of the cells, particularly with regard to mycoplasmas, should not be omitted. In our opinion, dyeing methods are not reliable enough in this case. Detection kits based on PCR (e.g. MycoSPY® or MycoSPY® Master Mix) are somewhat more complex but extremely reliable.
Mycoplasmas
Mycoplasmas are a serious problem in cell biology research. They are extremely widespread and are often not detected.
Mycoplasmas are bacteria which are not killed by conventional antibiotics like penicillin or streptomycin and are not visible under a microscope. Mycoplasmas are detected by the cell via the innate immune system. The result is that the cells take a defensive status that makes transfection difficult. The cells also proliferate more slowly than usual, which is another inhibitor of successful transfection. Overall, mycoplasmas can cause transfectability of cells to decrease by up to 95%.
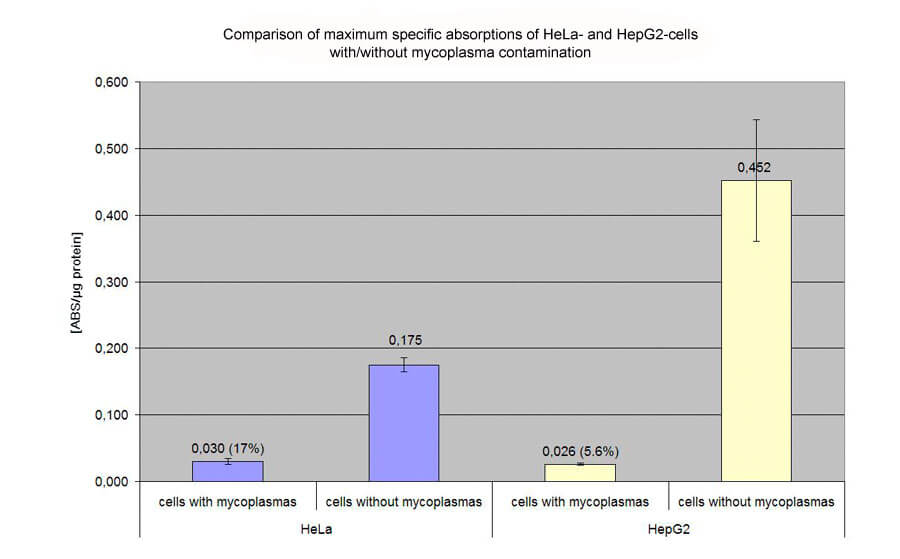
Biontex advises against the general prophylactic use of antibiotics directed against mycoplasma because this would promote the formation of resistant mycoplasma strains. If the personnel is the main source of contamination with bacteria and mycoplasmas, the antibiotics would first destroy all microorganisms. If resistance to bacteria developed, the bacteria would relatively quickly overrun the cell culture and thus flag up the contamination. This is not the case in the formation of resistant mycoplasma, which would not overrun the cell culture.
Proliferation of cells in plasmid transfection
Unlike RNA transfection, which is independent from cell division and proliferation because the cytosol is the location of effect, a protein coding DNA has to enter the cell nucleus to come into effect. Since no successful method has yet been established for actively transporting plasmids into the cell nucleus, plasmids rely on cell division to enter the nucleus. This phenomenon is also referred to as "nuclear barrier." Slow- or non-dividing cells (e.g. neurons) are correspondingly difficult to transfect. If the cell does not divide, access of the plasmids to the cell nucleus is largely blocked. However, there are indications that plasmids with corresponding sequences can be transported into the cell nucleus via a "piggy-back" mechanism of transcription factors.
In order to achieve optimum results with regard to protein yield, e.g. for the production of viruses, antibodies or other proteins, the greatest possible cell proliferation must be ensured at the time of lipoplexing.
If this relationship between proliferation and the success in transfection with plasmids is known, transfection efficiency can be increased.
Medium and serum
First of all, care should be taken over the right choice of medium. Literature frequently proposes a medium for a cell type which does not promote the proliferation of the cells in an optimal manner. Sometimes alternative media (often with higher glucose concentration) may be found that yield better proliferation.
The quality of the serum can also have an effect on the rate of proliferation. Again, it should be ensured that the serum meets the quality requirements.
Number of seeded cells
Ideally, cells of the same type follow the same growth curve. This is divided into a lag phase with low proliferation (also known as “latency phase”), and a log phase with logarithmic growth. As explained above, it is advantageous to add the lipoplexes at the beginning of the log phase.

Contrary to widespread opinion, the log phase begins when cells cover nearly all of the growth surface. This is because the concept of "confluency," in which cells stop growing because of contact inhibition, leads to the assumption that cells which are in contact shut down growth. However, conditions in practice are different, as can be seen in the following pictures.

Therefore, the aim should be to seed as many cells as necessary to reach the log phase at the point of time when the lipoplex/polyplex is added. This results in the highest rate of transfected cells and the highest protein yield. Since about 48 hours pass before maximum expression of the proteins takes place, however, the result is a relatively densely grown cell culture.
Unfortunately, many cells also depart from this growth behavior. They may proliferate faster, slow down, or show a growth curve with a different course.
Although creating a growth curve for your own cells requires some efforts, it can pay off if you want to get optimal transfection results.

If the aim is maximum transfection efficiency and a correspondingly high number of cells is selected, it may happen - especially in rapidly proliferating adherent cells - that the culture enters the "over-confluent" region, in which some of the cells become detached and apoptotic. Of course, the cells are also stressed by transfection with the high levels of nucleic acid necessary for maximum transfection efficiency.
Thus, transfected cells seem to detach rather than untransfected ones. Transfecting with plasmids coding for GFP often allows these cells to be detected microscopically in the cell supernatant.
As a rule, these cells are predominantly no longer vital, but can contribute to protein or virus yield. If the cultures are rinsed before the harvest or the assay, only partial results are obtained.
With respect to microscopic or various other cell biology studies, a dense stressed culture after transfection is often not a desirable result. At the time of transfection measurement, the aim is to achieve relatively loose growth on the growth surface with cell vitality as high as possible.
This can be achieved by seeding the cells with a correspondingly smaller number of cells at the beginning of transfection, and then optimizing the nucleic acid quantity and the nucleic acid quantity/reagent ratio.
However, transfection rates here are expected to be significantly lower due to the lower proliferation. Alternatively, of course, a dense transfected culture can also be "diluted," as it were, by subculturing.
If you are dealing with cells that divide slowly or not at all, it may be advisable to consider transfection with mRNA. In vitro transcription systems which can be used to prepare corresponding RNA are commercially available.
Optimization parameter: Nucleic acid/reagent ratio and lipoplex/polyplex amount
The amount of lipoplexes / polyplexes must be adapted to the number of seeded cells. The cell type determines the level of nucleic acid that is tolerated. Too high amounts of lipoplex / polyplex lead to toxic or apoptotic effects while too low amounts lead to lower transfection efficiencies. In fact, it is usually the nucleic acid released in the cell that has a negative effect on the vitality of the cells rather than the transfection reagent.
Lipoplexes/polyplexes can be prepared with varying amounts of nucleic acid and transfection reagent. The decisive factor for transfection activity is that the resulting complex has a net positive charge. However, complexes with different compositions show different transfection efficiency depending on the cell type used. Therefore, it is necessary to perform an optimizing procedure for every single cell type for use with a specific transfection reagent. As a rule, the manufacturers recommend reasonable optimization parameters.
Optimization of the nucleic acid/reagent ratio and the optimum lipoplex/polyplex amount or the optimum nucleic acid quantity for the previously determined cell number are usually carried out simultaneously, since these parameters are not completely independent of one another. The manufacturers' manuals often contain information on the quantities of nucleic acids for different cell culture vessels. Furthermore, ranges for nucleic acid/reagent ratios are usually indicated. The stated ranges are to be understood as empirically determined parameters within which optimal results are probable. It is important to keep in mind that the ranges for the nucleic acid quantities refer to cell densities that allow the maximum transfection efficiency. If lower cell densities are desired, these aspects have to be adapted.
It can be assumed that the stability of the lipoplexes is adjusted over the nucleic acid/reagent ratio. The more reagent (lipid) is used, the more stable the lipoplex. It must be understood that the lipoplexes or the nucleic acids are degraded by the cells, e.g. by nucleases located in the cytosol, and therefore have a limited life.
Different scenarios affect the stability of the lipoplexes in different ways:
If the site of action of the nucleic acid is the cytosol (siRNA / mRNA), results will benefit from a rapidly achieved high concentration of the nucleic acid in the cytosol. Accordingly, the lipoplex should be positively charged to allow endocytosis but otherwise be less stable to allow rapid release of the nucleic acid into the cytosol.
If the site of action of the nucleic acid is the cell nucleus (plasmids), the ratios can be different. Cells that split slowly benefit, for example, when the lipoplex is more stable, so that lipoplex or free nucleic acid is still present in later cell divisions.
Note: Since the rate of proliferation in a culture also depends on how many cells were seeded before transfection, the optimal amount of nucleic acid and the nucleic acid: reagent ratio can change if the number of seeded cells changes!
Optimal measurement time
Depending on the type of cell, reporter, or experiment, the time span for an optimum result between transfection and measurement may differ. Reporter GFP and luciferase have slightly different half-lives. The optimum time, i.e. the maximum protein expression, is here generally still about 36 to 48 hours after plasmid transfection. When mRNA is used this period is significantly shorter (about 16-24 hours). Suitable time periods for knockdown detection by means of siRNA depend on the analysis method: mRNA quantification (RT-qPCR) or protein quantification. When the analysis is performed on protein levels, the half-life of the protein is a major factor.
Construction of a successful transfection experiment
A successful transfection experiment is therefore constructed as follows:
- Ensure the quality of the nucleic acid
- Ensure cell health
- For plasmid transfection, optimize cell proliferation
- Specify the number of cells to be seeded (depending on the objective of the experiment)
- Optimize nucleic acid amount and nucleic acid/reagent ratio simultaneously
- Evaluate the experiment at the optimum measuring time
Adhesion to plastic surfaces and aging of lipoplexes
In principle, charged molecules such as nucleic acids and cationic lipids or polymers tend to adhere to glass and plastic surfaces. While the loss of mass resulting therefrom is negligible in the case of highly concentrated solutions, the conditions for dilute solutions used for lipoplex formation are different. In our experience this effect is unavoidable. Polypropylene is still the best of the available materials for lipoplex formation vessels (before polystyrene and glass). Care should therefore be taken to leave the diluted solutions in the vessels for as short a time as possible.
Lipoplexes also tend to adhere to plastic surfaces and, additionally, to "age." This means that the lipopolexes aggregate into larger units, which can not be absorbed by the cells in endocytosis, and this reduces their ability to transfect. Thus, after lipoplex formation you should proceed with the work as quickly as possible.
Up & downscaling
Due to the tendency of lipoplexes to adhere to plastic surfaces, transfection experiments cannot simply be transferred to differently sized culture vessels based on the proportionality of the growth surfaces. In a 96-well plate, the free plastic surface of the cylindrical side walls is significantly larger in relation to the growth area than in a 6-well plate. This means that much more lipoplex is lost at the side walls of a 96-well plate than of a 6-well plate. Most manufacturers of transfection reagents therefore recommend quantities given in the manual for up- and downscaling. Since the charge of the lipoplexes and thus their adhesion behavior, depends on the composition, these data can only be a rough approximation. The best strategy is to re-optimize the transfection experiment when changing the format.
Stable transfection
For stable cell transfection, the few cells which have randomly integrated the plasmid DNA into their genome must be selected using a selection antibiotic. For this purpose, the plasmid must bear the corresponding resistance to the antibiotic. The probability of random integration can be increased by linearizing the plasmid. During selection, the best strategy is to increase the selection pressure slowly.
The innate immune system
The innate immune system plays an important role in transfection processes. Cells can basically detect all current nucleic acids and distinguish between "foreign" and "own" by means of endosomal receptors (e.g. toll-like receptors) and also cytosolic receptors. If an alien nucleic acid is detected, the cell uses signal transduction cascades to convert the protein expression and create a defense. In addition messenger substances - particularly interferon-β - are released, so that cells which are not in direct contact with the nucleic acid also build up a defense.
However, the expression of the innate immune system differs from cell type to cell type. In addition to differences in proliferation, different transfectability of different cell types may be in evidence.
In particular, suspension cells and primary cells are more difficult to transfect than adherent cell lines.
Since suspension cells derive from immune cells, as long as non-adherent cells have been adapted to suspension conditions, these cells are logically characterized by a pronounced immune system.
Due to corresponding selection pressure, primary cells are also equipped with a better innate immune system than cell lines which are no longer exposed to this selection pressure in the cell culture. As a good example, HEK293 cells showed no detectable nucleic acid-detecting receptors and are considered to be easily transfected cells. The role of the innate immune system for transfection with synthetical carrier systems led us to the development of the K2® Transfection System and later on to the K4® Transfection System.
Freeze/thaw procedure
Long-term storage of liposome solutions at 4°C or RT can result in liposome coagulation. Liposome size distribution is thus shifted towards larger liposomes, which can impair transfection efficiency. As described in the manual for the METAFECTENE series (METAFECTENE®, METAFECTENE® PRO, METAFECTENE® SI) and the K2® Transfection System/K4® Transfection System, such liposome solutions can be restored by a freeze/thaw cycle. For this purpose the reagent must be frozen overnight at -20°C and subsequently thawed and stored again at 4°C. The freeze/thaw cycle does not harm the molecules of the reagent and can be repeated any number of times. We recommend this procedure before first use and subsequently every 4 weeks.
Edge effects
In multiwell plates, poorer transient results are often observed at the edges of the plates than in the middle at exactly the same conditions. This is because the outer wells are more affected by evaporation effects than the inner wells. As a result, the salts are concentrated in the medium and the osmolality changes. The cells do not suffer and proliferate as effectively. However, as a consequence the transfection efficiency decreases in this wells. Regular monitoring of the water level (for humidity) in the incubator can usually remedy the problem. In addition, in case of doubt, it is better to work with more medium than less.
Microscope-FACS-GFP
One of the most frequently used reporters is "green fluorescent protein," or GFP. If a cell is transfected with a corresponding plasmid encoding GFP, GFP is produced in the cytosol of the cell. This allows transfected cells to be identified by means of a fluorescence microscope or FACS. However, the results of these analyzes depend on various factors.
wtGFP, eGFP and TurboGFP
"Wild type" GFP is now hardly used, since a significantly more strongly emitting GFP is available in the form of "enhanced" GFP. GFP was originally derived from a jellyfish species (Aequorea victoria). Replacement of a few amino acids in the chromophore significantly increased its emission. This eGFP was so successful that it almost completely displaced the "wild type" GFP. However, there are now many eGFPs, some protein of which is also derived from other organisms, e.g. pancella (Pontellina plumata), which show different emissivity. Copepod variants are often referred to as copGFP or TurboGFP, but are frequently only declared as eGFP. Due to their high luminosity, we recommend this variant as they are the most sensitive way of detecting transfection with GFP. On account of these different sensitivities, the measured transfection rates differ significantly as a function of the GFP variant. Also, comparison of different results must be applied with care where different promoters are involved.
This also applies in a broad sense to the measurement method. While a FACS sensitively detects every cell with fluorescence above that of self-fluorescence, the sensitivity of the photochip or the human eye is key in microscopic counting. Correspondingly, the highest transfection efficiency will be determined using FACS, while the lowest will be found by counting with the microscope without digital support.
It is therefore possible that seemingly very different results are in fact relatively similar. Conversely, apparently comparable results generated with the same plasmid may indeed be very different, namely when working with the microscope and a camera with exposure time set to "automatic" or "auto exposure" by means of software. Here, relatively different results can be evened out, since weakly-emitting cultures with high exposure time and strongly emitting cultures with low exposure time are photographed.
Special case: Suspension cells
Adherent cells are embedded in an extracellular matrix created by the cells themselves. This matrix is permanently formed and degraded by processes of endo- and exocytosis. It is assumed that most lipoplexes/polyplexes bind to negatively charged components of these extracellular matrices and finally enter the cells through endocytosis.
Classic suspension cells do not have an extracellular matrix (this does not apply to cells adapted to suspension conditions). As a result, the uptake of lipoplexes/polyplexes is low; this can be counteracted with drastically increased amounts of lipoplexes. In addition, medium supplements like transferrin and insulin, which are internalized in the lipoplexes/polyplexes if present during formation, can increase the uptake rate by means of receptor-mediated endocytosis, as every cell has receptors for these molecules.
Comparison of experiments
Different test results from exactly the same test parameters are not uncommon. Results of transfection experiments show a considerable fluctuation range.
This is essentially due to the fact that the physiological status of the cells is never the same. If the cells are trypsinized and sown to prepare a transfection experiment, they undergo a certain stress level, which can be different in another harvesting process.
Furthermore, cell number determinations are associated with a high level of error, which may cause achievement of the log phase to shift by critical hours; this can have a fundamental influence on transfection results when working with plasmids.
In general, test results are comparable when the experiments were carried out with the same cell suspension, which was usually sown on the same culture vessel (e.g. 6-well plate).
NOTE: This guide is for Biontex products only.